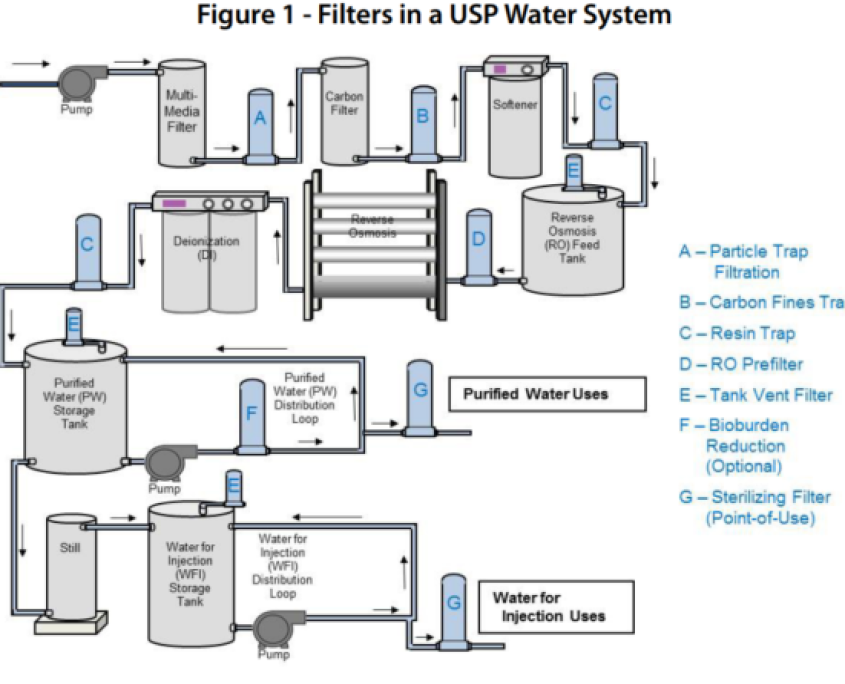
Water Supply
The Water supply in cleanrooms and pharmaceutical industries is a combination of equipment and purification processes designed to convert raw water into various grades of high-purity water, such as RO Water, Purified Water (PW), and Water for Injection (WFI).
This system removes physical, chemical, and microbial impurities through multiple stages, including pre-filtration, activated carbon filtration, reverse osmosis (RO), deionization (EDI or DI), UV or ozone disinfection, and finally multi-effect distillation.
Precise design, continuous quality monitoring, and constant water recirculation through stainless steel pipelines are essential to ensure consistent, sterile, and pharmaceutical-grade water quality.
2. Purified Water (PW)
Definition:
Pharmaceutically purified water that is chemically and microbiologically clean but not suitable for direct injection into the human body.
Application:
Used for cleaning equipment, preparing non-injectable pharmaceutical solutions, and manufacturing oral or topical products.
Production Process:
RO water undergoes final polishing steps such as:
- Ion removal using EDI or DI resin systems
- Disinfection using UV or ozone
- Storage and recirculation in stainless steel tanks to prevent microbial growth
1. RO Water (Reverse Osmosis Water)
Definition:
Water produced by the Reverse Osmosis (RO) process, where suspended solids, dissolved salts, and most chemical contaminants are removed from raw water.
Application:
Used as the initial step in producing higher-purity water (PW and WFI) or for non-critical cleaning purposes.
Production Process:
Raw water passes through sand and carbon filters to remove particles and chlorine, then enters RO membranes where salts and impurities are separated.